When European hospitals buy generic medicines, they don’t just pick the cheapest option off a shelf. They go through a formal, tightly regulated process called tendering. This isn’t just bureaucracy-it’s a system designed to save money, ensure quality, and keep the market fair for suppliers across 27 countries. For pharmacies, manufacturers, and public health systems, understanding how this works isn’t optional. It’s how billions in public funds are spent every year.
How European Tendering Works in Practice
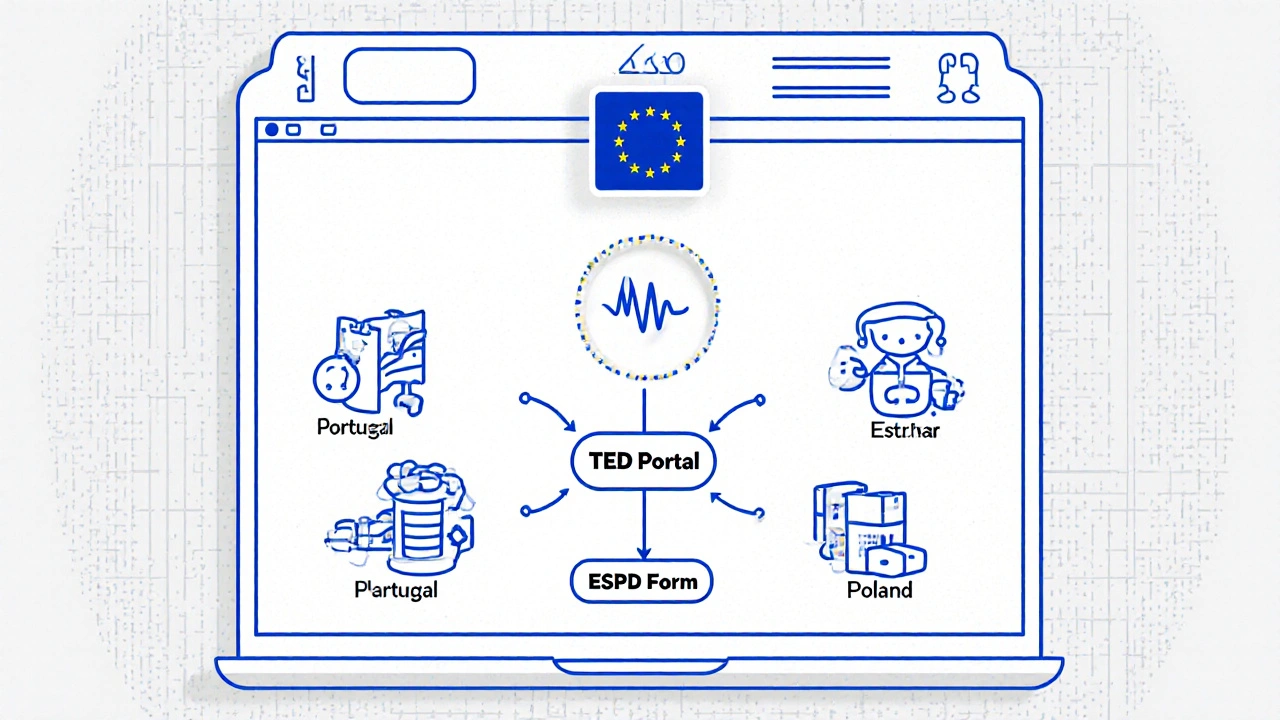
Every public contract above €25,000 in the EU must follow rules laid out in Directive 2014/25/EU. That means when a regional health authority needs to buy 10 million tablets of generic metformin, they can’t just call their usual supplier. They must advertise the need publicly, usually on Tenders Electronic Daily (TED), the EU’s central tender portal. Any company in the EU can respond-even a small firm in Portugal or a startup in Estonia.
The most common method is the Open procedure. It’s simple: anyone can submit a bid. About 45% of all EU public tenders use this route. It’s great for competition, but it also means authorities get hundreds of bids to sort through. For generic drugs, where the product is identical across brands, this often comes down to price. But not always.
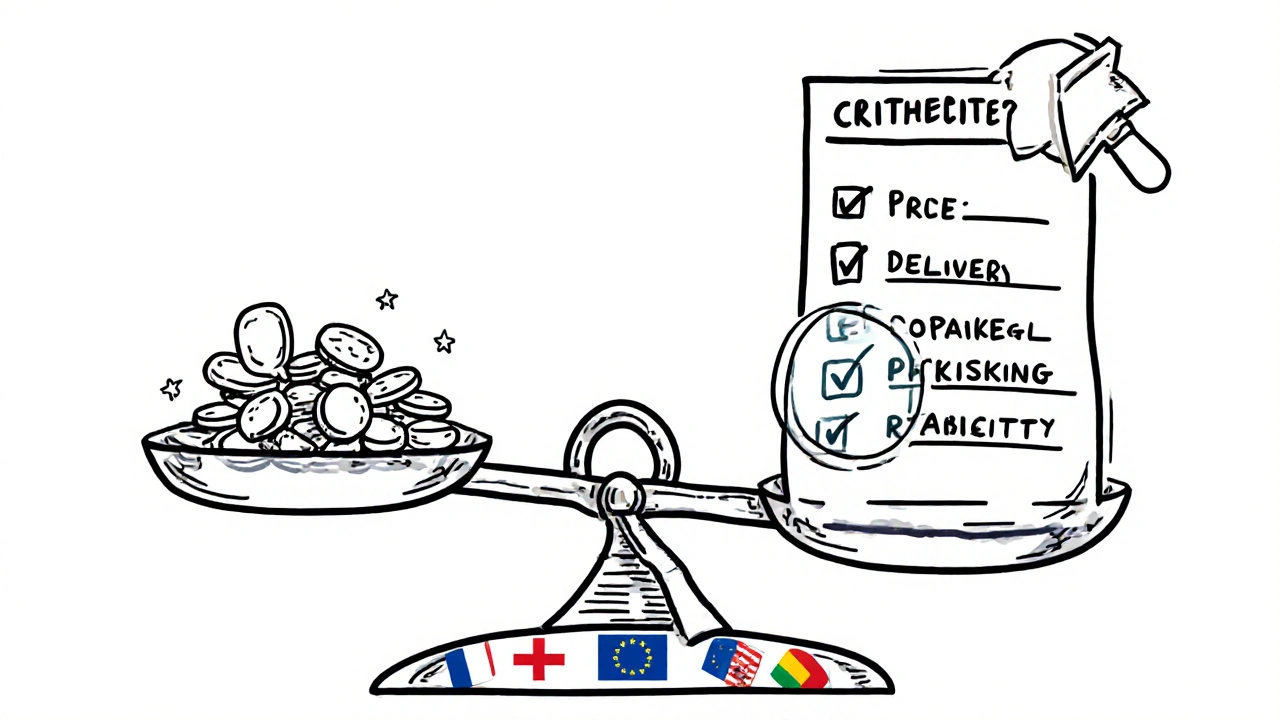
That’s where MEAT-Most Economically Advantageous Tender-comes in. Instead of just picking the lowest price, authorities score bids on a mix of factors: price, delivery reliability, packaging safety, environmental impact, and even supplier track record. A 2023 study found that health authorities using MEAT saw 15.7% more innovation in their purchases, like blister packs with child-safe caps or carbon-neutral logistics. For generics, that might mean choosing a supplier who guarantees stock for 12 months over one who offers 5% less but might run out in six.
The Four Main Tendering Procedures
Not all purchases are the same. The EU gives authorities four main ways to run a tender, each suited to different needs.
- Open procedure: Everyone can bid. Best for simple, high-volume purchases like common generics. High competition, high paperwork.
- Restricted procedure: Suppliers must first apply to be invited. Only pre-qualified bidders submit final offers. Used when authorities want to reduce volume but still keep it fair. About 35% of tenders use this.
- Competitive Negotiated procedure: Used when the product is complex or there’s only one supplier with the right tech. Rare for generics, but common for specialty drugs or digital health systems.
- Competitive Dialogue: For projects where the authority doesn’t yet know exactly what they need. Suppliers help design the solution. Used in digital health projects, not typically for off-patent pills.
For generic medicines, Open and Restricted procedures dominate. Why? Because the product is standardized. The active ingredient is the same. The difference is in packaging, delivery times, and price.
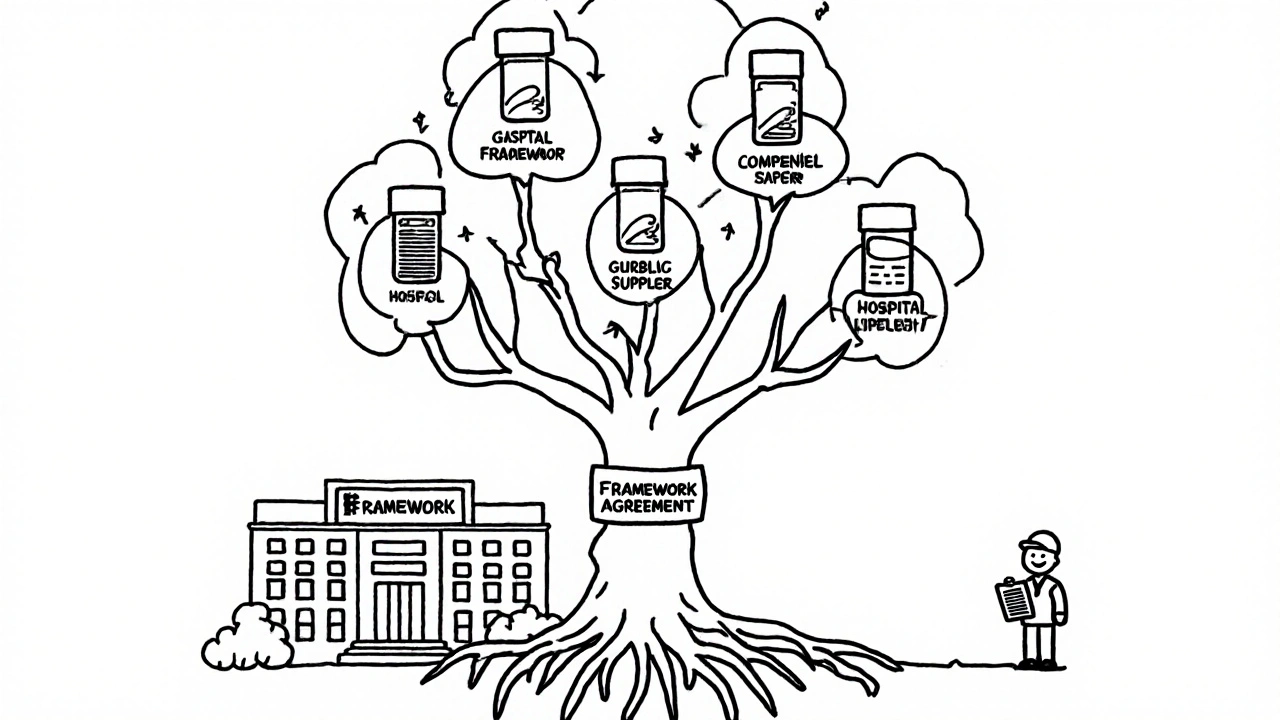
Framework Agreements: The Hidden Backbone of Generic Supply
Most public health systems don’t buy generics one-off. They set up Framework Agreements. Think of it like a pre-approved list of suppliers. Once a hospital signs up with a framework, they can order from any of the approved vendors without running a new tender each time.
There are two types: single-supplier and multi-supplier. Multi-supplier is far more common-and smarter. In a multi-supplier framework, say with five approved generic metformin suppliers, the hospital runs mini-competitions every time they need a refill. That keeps prices low and prevents lock-in.
But it’s not perfect. A 2023 survey of 1,200 EU suppliers found that while 60% of companies that won a framework agreement saw their bidding costs drop by 60%, 38% said they got so few actual orders that the initial effort wasn’t worth it. One French SME spent 8 months preparing documents to join a national framework-then got only two orders in 18 months. That’s a risk small suppliers take.
Why Some Tenders Fail-and How to Avoid It
Not every tender works. The European Court of Auditors found that 37% of Competitive Dialogue tenders in 2022 had poor documentation. In Spain, a €180 million rail signaling contract was canceled after 18 months because the technical specs were too vague. The same happens in health.
One major reason? Ambiguous requirements. A 2022 analysis showed that 68% of supplier complaints in cross-border tenders came from unclear product specs. For generics, that might mean a tender asking for “high-quality packaging” without defining what that means. Is it child-safe? Tamper-proof? Recyclable? Without clear criteria, suppliers guess-and end up bidding wrong.
Successful authorities fix this by doing pre-tender market consultations. They talk to suppliers before publishing the tender. Ask: What’s realistic? What packaging do you use? Can you deliver 500,000 units by March? This reduces complaints by 34% and cuts procurement time by 22%.
The Digital Shift: How Tech Is Changing the Game
Europe is moving fast toward fully electronic procurement. In 2022, 76% of all public tenders were done online-up from 39% in 2016. The European Single Procurement Document (ESPD) replaced stacks of paper with a simple online form. Now, suppliers can self-declare their financial health, tax compliance, and past performance. No need to send certified bank letters or notarized copies.
That’s a game-changer for small businesses. Before, a Polish generic drug maker might spend €5,000 just on paperwork to bid on a German hospital contract. Now, it’s under €200-and most of that is time, not money.
By 2027, the EU aims for 95% of tenders to be electronic. Some countries are ahead: Nordic nations hit 92% e-tendering. Others lag-Southern Europe is still around 43%. But the trend is clear: paper is dying.
Proportionality: The EU’s Secret Weapon
One thing that makes the EU system unique is the proportionality principle. Authorities can’t demand ridiculous things just to scare off small suppliers. For example: if you’re buying €500,000 worth of generic insulin, you can’t require a supplier to have €10 million in annual revenue. That’s illegal.
This rule levels the playing field. It’s why a small Czech lab can win a contract in Sweden. It’s why generics from Eastern Europe are now common in Western hospitals. Without proportionality, big players would dominate by default.
But enforcement is uneven. The European Court of Auditors warned in 2023 that 30% of member states still slip-adding hidden requirements that favor local firms. That’s why cross-border bidding remains low: only 7.3% of total tender value comes from suppliers outside the buyer’s country. Northern states like Denmark and Finland have 14% cross-border activity. Southern states? Just 3.8%.
What’s Next? Sustainability, AI, and Strategic Autonomy
By 2025, 85% of high-value EU tenders will include sustainability criteria. That means for generics, suppliers will be scored not just on price, but on:
- Carbon footprint of manufacturing
- Use of recyclable or biodegradable packaging
- Energy efficiency of logistics
Some countries are already ahead. The Netherlands now requires all public health tenders to include a circular economy score. Germany’s public hospitals are testing biodegradable blister packs.
And AI is coming. Pilot programs in France and Finland use algorithms to score bids automatically. They check for price consistency, delivery timelines, and past performance. Results? 30% faster evaluations-with 99.2% accuracy compared to human reviewers.
But the biggest shift might be geopolitical. With supply chain risks from Asia and drug shortages during crises, the EU is now talking about “strategic autonomy.” That means favoring suppliers within the EU or allied countries for critical medicines. It’s not protectionism-it’s resilience. And it’s already creeping into tender rules.
What Suppliers Need to Know
If you’re a generic drug maker or distributor trying to win EU contracts, here’s what actually works:
- Register on TED and set up email alerts for your product codes (CPV codes). Misclassifying your product gets your bid rejected-23% of rejections in 2022 were due to this.
- Study the MEAT criteria. Don’t just undercut on price. Highlight reliability, packaging innovation, and delivery speed.
- Join a multi-supplier framework. It’s the best long-term bet-even if it takes months to qualify.
- Use the ESPD. It’s free, online, and accepted across the EU.
- Don’t ignore pre-tender consultations. If the authority invites suppliers to a meeting, go. It’s your chance to shape the tender.
Companies with certified procurement professionals (CPP) report 28% higher win rates. It’s not magic-it’s knowing the rules inside out.
Final Reality Check
The EU tendering system isn’t perfect. It’s slow. It’s complex. It can be frustrating for small businesses. But it’s also the most transparent, fair, and effective public buying system in the world. It’s why Europe can buy generic drugs at 60% lower prices than the U.S. It’s why patients get the same medicine, regardless of whether they live in Rome or Riga.
For suppliers, it’s a challenge. For public health systems, it’s a lifeline. And for patients? It’s the quiet engine behind affordable, reliable medicine.
What is the most common tendering procedure for generic medicines in the EU?
The Open procedure is the most common, used in about 45% of EU public tenders. It allows any supplier to submit a bid without prior qualification, which works well for standardized products like generic medicines where competition is based mainly on price and delivery terms.
How does MEAT differ from lowest-price-only bidding?
MEAT (Most Economically Advantageous Tender) evaluates bids on a combination of price and non-price factors like quality, reliability, delivery speed, packaging safety, and environmental impact. Lowest-price-only focuses only on cost. MEAT helps avoid hidden risks-like stockouts or poor packaging-and leads to better long-term value, especially for critical medicines.
Can small generic drug companies compete in EU tenders?
Yes. The EU’s proportionality principle bans unfair requirements like minimum revenue thresholds. Small companies can win contracts by focusing on reliability, clear documentation, and joining multi-supplier framework agreements. Over 40% of successful bidders in EU health tenders are SMEs.
What is the European Single Procurement Document (ESPD)?
The ESPD is a standardized online form that allows suppliers to self-declare their eligibility-like financial standing, tax compliance, and past performance-without submitting physical documents. It cuts administrative costs by up to 40% and is now mandatory across all EU member states for tenders above €25,000.
Why are framework agreements important for generic procurement?
Framework agreements pre-select approved suppliers, allowing health authorities to make faster, repeated purchases without running new tenders. Multi-supplier frameworks create competition even after selection, keeping prices low and supply secure. They’re the backbone of efficient generic drug procurement across Europe.
All Comments
Mariam Kamish November 25, 2025
This is so much paperwork for pills. 🥱 Why can't we just buy the cheapest and move on?
Patrick Goodall November 26, 2025
They say it's fair but let's be real - the big pharma lobby wrote these rules. MEAT? More like Most Expensive Advantageous Tender. 🤡 The EU’s just making sure your grandma’s meds come in fancy packaging while the real suppliers get crushed.
Manish Pandya November 27, 2025
This is actually really well explained. The MEAT system makes total sense - price alone doesn’t guarantee you won’t run out of stock in winter. And the ESPD? Huge win for small labs. Finally, someone’s thinking about fairness, not just cost.
Kaylee Crosby November 28, 2025
You guys are underestimating how powerful this system is. Small suppliers can actually win here - I’ve seen it happen. The key is not to fight the process, just learn it. Use the ESPD, join frameworks, and don’t skip those pre-tender chats. You’ll thank yourself later 💪
Adesokan Ayodeji November 29, 2025
Honestly this is one of the few times I’ve seen a public system work the way it’s supposed to. In Nigeria we struggle to get basic meds on time, and here they’ve built a whole structure to make sure no one gets left behind. The framework agreements? Genius. Even if it takes months to get in, once you’re in, you’re in for the long haul. Keep pushing, small suppliers - you’re not invisible here.
Karen Ryan December 1, 2025
I love how this system respects cultural differences too - like packaging safety standards that work for both rural Romania and urban Stockholm. It’s not just about money, it’s about dignity. 🌍❤️
Terry Bell December 2, 2025
Kinda makes you wonder why the US still does this chaotic free-for-all. Here, the system’s clunky but it’s designed to protect people, not profits. Maybe we need more of this… and less of that Wall Street chaos.
Lawrence Zawahri December 2, 2025
This is all a scam. TED? ESPD? MEAT? It’s all just a front for the EU to control the drug supply. Next thing you know they’ll mandate which color the blister packs are. They don’t care about patients - they care about control. The real winners? The bureaucrats who get paid to review paperwork. 🕵️♂️
Benjamin Gundermann December 4, 2025
Look, I get it - the EU wants to be the world’s moral compass. But let’s be honest, this whole system is just Europe’s way of saying ‘we’re better than you.’ Meanwhile, America’s system is messy but it gets drugs to people faster. And don’t even get me started on how they treat American suppliers trying to bid. It’s like they want us to fail. We built the modern pharmaceutical industry - now they’re putting on airs like they invented it.
Rachelle Baxter December 6, 2025
The fact that you’re even surprised that small companies can win is concerning. This isn’t a gift - it’s a legal requirement. The proportionality principle is non-negotiable under EU law. If you’re still complaining about bureaucracy, you’re either not reading the directives or you’re just resistant to fairness. 🙄
Dirk Bradley December 7, 2025
The institutional architecture underpinning the EU’s public procurement framework represents a paradigmatic shift toward technocratic governance predicated on transparency, accountability, and supranational regulatory harmonization. One cannot underestimate the epistemological weight of the ESPD as a decentering mechanism for bureaucratic opacity.
Emma Hanna December 7, 2025
You didn’t even mention the environmental impact of shipping pills from Poland to Portugal. And what about the carbon footprint of all those PDFs? And the fact that 38% of SMEs get almost nothing in return?! This system is a disaster disguised as a solution. And you’re just… nodding along?!
Valérie Siébert December 8, 2025
AI scoring bids? YES. That’s the future. No more humans guessing what ‘high-quality packaging’ means. Let the algorithm pick based on real data - delivery latency, return rates, past compliance scores. This is procurement 2.0. Get on board or get left behind 🚀
Jacqueline Aslet December 10, 2025
It’s ironic, isn’t it? All this complexity to ensure fairness… yet only 7.3% of bids come from outside the buyer’s country. So much for ‘European unity.’ It’s just localism with a fancy acronym.
Caroline Marchetta December 12, 2025
I spent 8 months preparing a bid… got one order. One. And now I’m drowning in paperwork I can’t afford. They call it ‘fair’? It’s a trap. They lure you in with the dream of a framework, then leave you to rot in the ‘approved but ignored’ pile. The EU doesn’t want small suppliers - it wants the illusion that it does.