Tag: pharmacokinetics
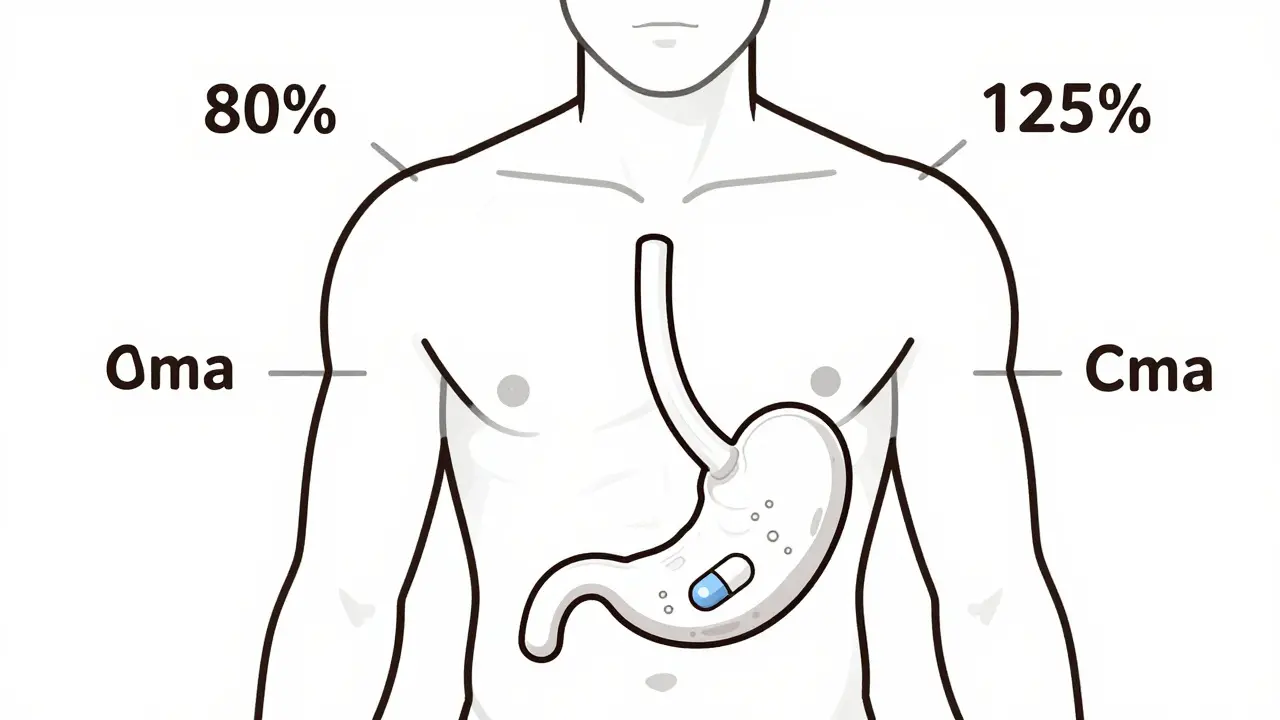
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
- Cheryl Moran
- January 27, 2026
- 12 Comments
The 80-125% rule ensures generic drugs work like brand-name versions by measuring how much drug enters your bloodstream. It's not about pill strength-it's about body absorption. Here's how it works.
read more