Tag: generic drugs
Authorized Generics List: Which Drugs Offer This Option
- Cheryl Moran
- February 8, 2026
- 15 Comments
Learn which prescription drugs offer authorized generics - identical to brand-name versions but at lower prices. Discover key examples, how they differ from regular generics, and how to find them.
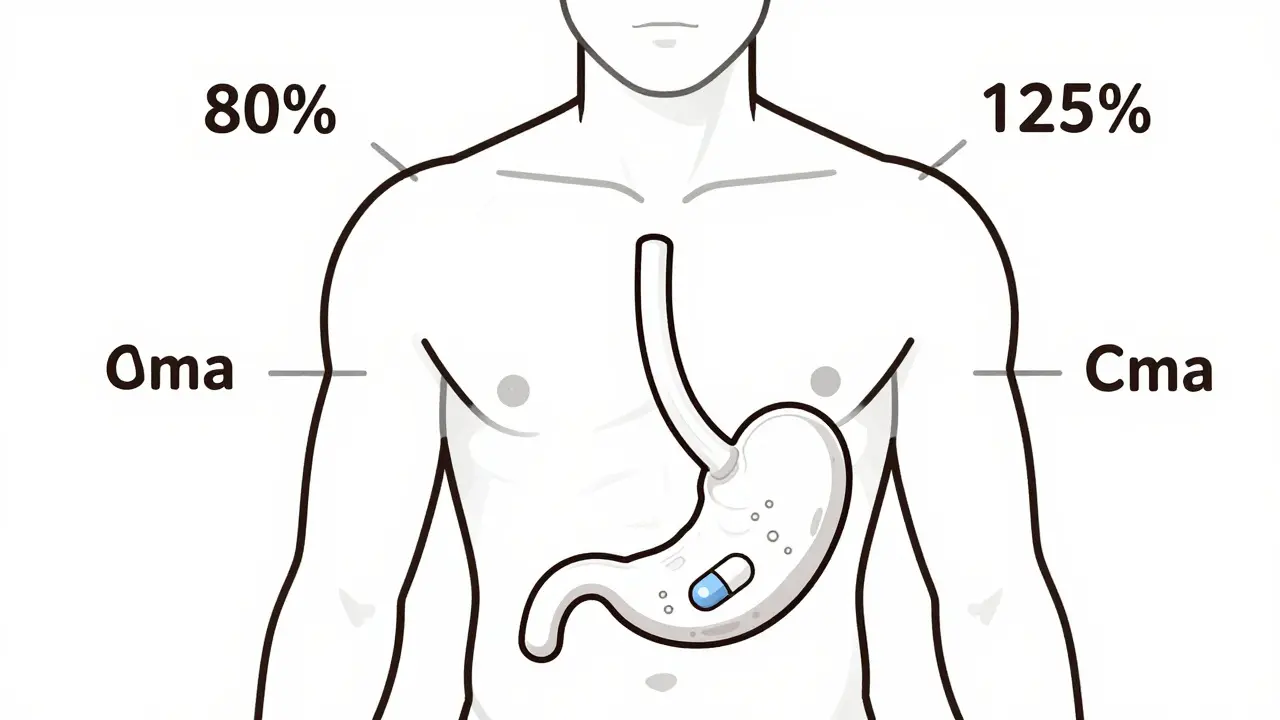
read moreThe 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
- Cheryl Moran
- January 27, 2026
- 12 Comments
The 80-125% rule ensures generic drugs work like brand-name versions by measuring how much drug enters your bloodstream. It's not about pill strength-it's about body absorption. Here's how it works.
read moreRare Adverse Events with Generics: When and How to Report Side Effects
- Cheryl Moran
- December 28, 2025
- 14 Comments
Learn when and how to report rare side effects from generic medications. Understand FDA reporting rules, what counts as serious, and why your report matters - even if you're just a patient.
read moreWhy Generic Medications Cost Less for Patients and Insurers
- Cheryl Moran
- November 14, 2025
- 11 Comments
Generic medications save patients and insurers hundreds of billions annually by offering identical effectiveness at a fraction of the cost. Learn how competition, FDA rules, and smart purchasing drive these savings.
read more